To begin with, let’s talk about the complexity of AKD sizing problem from the perspective of interaction. We know that the interaction of wet end chemistry is divided into three types: chemical reaction, colloidal
interaction, and physicochemical interaction. The special feature of AKD sizing is that not only these three interactions are involved, but also the chemical reactions and interactions ar
e particularly complicated. Based on the information obtained, we think the chemical reactions that should be considered when AKD sizing are as follows:
(1) The reaction between AKD and water (the hydrolysis reaction of AKD, see Figure 1);
(2) The reaction between AKD and fiber (see Figure 2);
(3) The reaction between AKD and starch emulsifier (the figure is omitted, it can be regarded as similar to Figure 2);
(4) The reaction of AKD with emulsification accelerator and other trace substances;
(5) Other reactions caused by AKD hydrolysis intermediate products;
(6) Other reactions caused by AKD polymer.
So, AKD sizing is very compli
cated from the perspective of interaction.
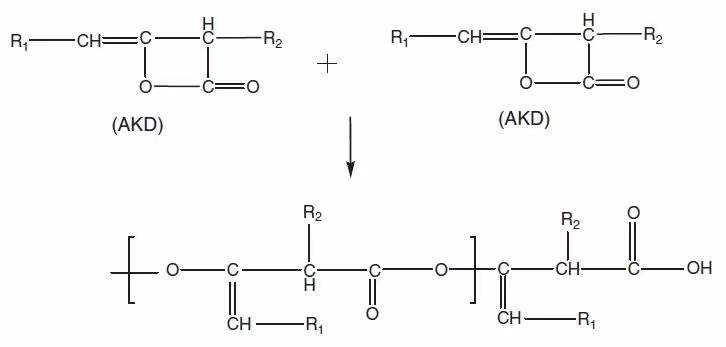
Figure 1. Schematic diagram of the hydrolysis reaction of AKD (before carbon dioxide removal is the beta-keto acid reaction intermediate)
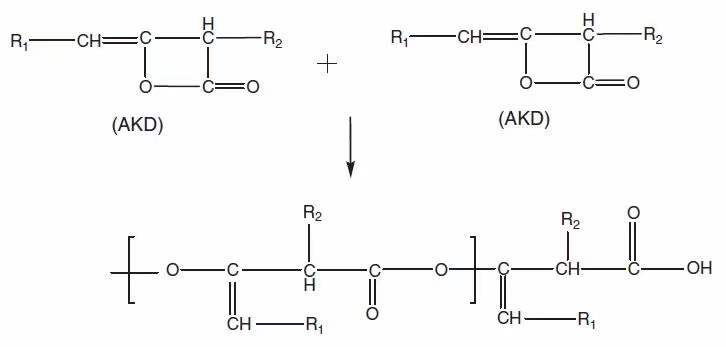
Figure 2. Schematic diagram of the reaction between AKD and cellulose (generating beta-ketoester)
Horizontally, if comparing AKD with the same category of actual wet end chemical additives such as dry strength agents and retention aids, since the latter generally does not involve real chemical reactions, and physicochemical interactions are basically not involved, you can better appreciate the complexity of AKD sizing.
However, most of the previous studieson the reaction mechanism of AKD only focused on reactions (1) and (2), it’s not surprising that the explanatory power is insufficient (the root causes of various problems such as insufficient sizing, disappearance of sizing, and reversal of sizing are unclear).
Let’s look at other reactions: there have been some good studies of reaction (3). Nevertheless, to think systematically, there is still a lot of work to be done (special note: we will correct this opinion at any time if it’s wrong). And reaction (4) should also be considered (at least not to be ignored), and its logic is actually the same as reaction (3), because emulsification promoters and other trace substances may also have active hydroxyl groups or amine groups that can react with AKD . Reaction (5) is the side reaction caused by the intermediate product of AKD hydrolysis. A simple example is that the intermediate product (beta keto acid) shown in Figure 1 combines with calcium ions, magnesium ions and other hardness components, resulting in the formation of deposits. Finally, reaction (6) is a large category, and its focus is on the AKD polymer that is not familiar to everyone. And the formation mechanism of this polymer is shown in Figure 3.
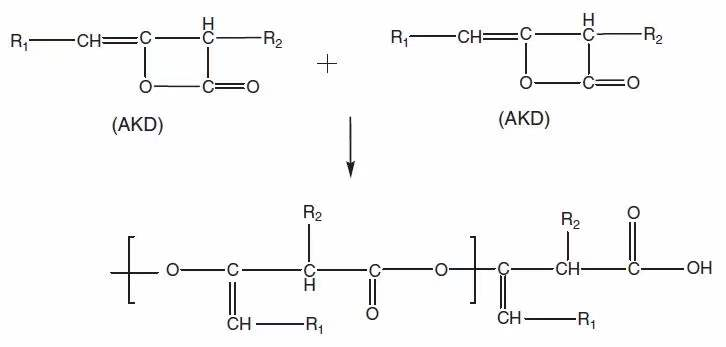
Figure 3. Schematic diagram of the formation of AKD multimers (may be produced when AKD raw materials are synthesized)
AKD polymers are mainly derived from AKD raw materials, and a small part may be derived from the application process of AKD (the conclusion is not certain, and we will leave a message to correct it if there is a clearer statement). As can be seen from Figure 3, the polymer has a carboxyl group, so it is also prone to other incidental reactions.
I believe that everyone now has a deeper understanding of the complexity of AKD applications than before, but new problems have followed–What should we do in the face of such complex problems?
The answer, of course, is to simplify reasonably (if you only consider the reactions (1) and (2) , that’s oversimplified and not reasonable).
One of the reasonable simplification methods is to classify the reaction products. In fact, the AKD that does not react with the fiber as shown in Figure 1 is called unbound AKD (unbound AKD, noted UAKD for short), and the AKD that reacts with the fiber as shown in Figure 2 is called bound AKD (bound AKD, noted BAKD for short). Of course, to be more rigorous, replacing the fiber with starch can also be divided into two parts: UAKD and BAKD. Measuring the content of UAKD and BAKD in paper separately should help to better understand the sizing mechanism of AKD.