介绍
内部施胶可以防止水性液体渗透到整个纸张。内部施胶是通过在流浆箱前向纸浆中添加材料来实现的,以阻止水渗透到最终的纸张中。施胶分子的非极性部分可以减缓水的渗透。施胶分子的反应性部分将其锚定在纤维表面。
表面施胶的原理不同,它发生在施胶压榨过程中,淀粉(或其他材料)会填充纸张的毛细孔,使水的渗透变得更加困难。淀粉不像内部施胶剂那样具有疏水性。
自19世纪初开始使用的明矾松香施胶,仅在pH值低于7左右时才有效。碱性施胶则发展于20世纪40年代和50年代。近年来,碱性施胶在美国广泛用于填充碳酸钙的印刷纸,这种纸张的pH值必须在7或更高,因为在酸性条件下,碳酸钙会分解成二氧化碳气体,导致纸张出现麻点。
纸张可能是:
- 硬质施胶纸(对液体渗透有很强的抵抗力,例如许多印刷和包装纸),
- 松散尺寸(对液体渗透的抵抗力较低,例如新闻纸),或
- 无施胶(毛巾、吸墨纸和卫生纸)。
内部尺寸确定有两种常用方法:
- 松香施胶必须在 pH 值为 4-6 时进行,
- 碱性施胶,在 pH 值为 8 或更高时使用烯基琥珀酸酐 (ASA) 或烷基烯酮二聚体 (AKD) 进行。
碱性造纸的优点包括:
- 由于没有残留酸,因此寿命长,
- 能够使用碳酸钙(一种明亮且廉价的填料),
- 更坚固、更不易碎的纸张,
- 减少对造纸机的腐蚀,
- 含有碳酸钙的再生纤维的问题较少。
硬木浆比软木浆更容易施胶。硫酸盐浆比亚硫酸盐浆、热机械浆或磨木浆更容易施胶。其原因在于,羧酸盐基团的数量是施胶的锚点。
松香内胶
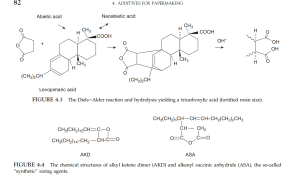
传统上最常见的内部施胶剂是松香(2-9 千克/吨或 4-20 磅/吨),用明矾(Al₂(SO₄)₃)沉淀,该工艺于 1807 年开发。通过狄尔斯-阿尔德反应,马来酸酐加成可强化松香酸及其同系物。
松香可用于:
- 盐形式(肥皂大小,pH 值 10–11),
- 糊状物(含 20–30% 水的固体盐),
- 游离酸形式(乳液大小),在稍高的 pH 值下有效。
尺寸顺序:
- 通常,先添加松香,再添加明矾。
- 如果先添加明矾,则称为反向施胶,适用于高钙水或碱性系统。
采用 ASA 或 AKD 进行内部施胶
在碱性条件下制造的纸张使用合成施胶剂,例如:
- AKD(例如 Aquapel) ——通过脂肪酸酰氯二聚化制备,
- ASA——由 C-16 至 C-20 烯烃与马来酸酐反应制成。
 重要提示:
重要提示:
- ASA 必须不与水发生水解才能保持其有效性。
- ASA 乳液应在酸性条件下(pH 3.5–4)短期储存。
- AKD 更稳定,可以稀乳液形式储存数周至 3 个月。
- 两者都必须用阳离子淀粉乳化。
乳化指南:
- ASA:淀粉冷却至<38°C,pH 值 4–4.5,
- AKD:淀粉溶液 pH 值为 5–7,最大施胶时特性粘度为 1.0–1.1。
合成浆料可能含有阳离子基团,以提高留着率。ASA 通常与 4-5 千克/吨明矾配合使用,可在机上快速固化。AKD 可能会受到明矾的抑制,需要 1-2 周的停机时间才能完全固化。
使用合成浆料的工厂面临的挑战:
- 光滑的纸张(摩擦系数低),
- 成形网寿命缩短,
- 压榨部采摘增加,
- 碳酸钙填充纸张的成形问题,
- 施胶压榨后上浆效果较差。